Nitrosamines in infusion bags: what does the FDA say and how can you protect yourself?
Background: What is N-nitrosodibutylamine (NDBA)?
Nitrosamines are chemical compounds known for their carcinogenic potential.
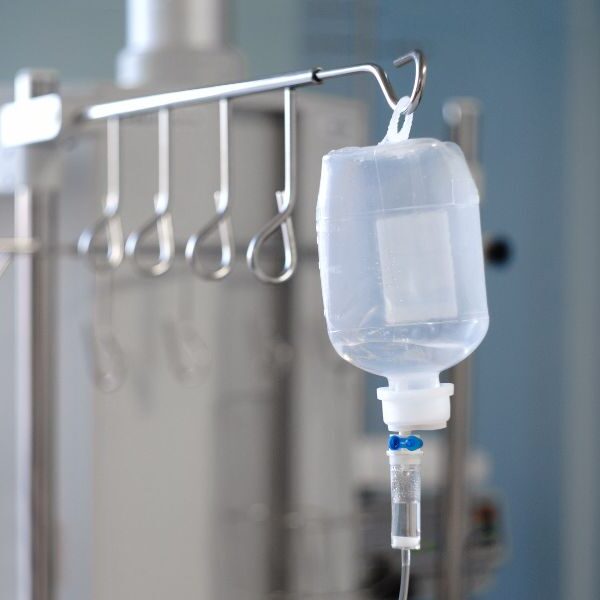
N-nitrosodibutylamine (NDBA) is a compound belonging to the nitrosamine family and may form during the manufacture or storage of certain pharmaceutical products.
The presence of NDBA in medicines packaged in infusion bags raises significant concerns for pharmaceutical manufacturers regarding regulatory compliance.
The recent FDA alert: key issues and dates
On 18 August 2025, the FDA confirmed the detection of NDBA in certain infusion bags. This announcement triggered a requirement for manufacturers to reassess their processes and materials in order to avoid any risk of contamination. The challenge is twofold:
- Patient safety: preventing exposure to potentially harmful substances
- Regulatory compliance: document and communicate to the authorities the measures taken to limit nitrosamines
Obligations for manufacturers
In response to this alert, manufacturers must implement several measures:
- Evaluation of packaging materials: films, outer bags, inks and other components must be analysed to detect any risk of nitrosamine formation.
- Ultra-sensitive analysis: nitrosamines are active at ultra-trace levels (ppb), requiring sophisticated analytical techniques.
- Documentation and traceability: all actions and results must be recorded and communicated to the competent authorities.
These steps are essential to comply with FDA requirements.
Ultra-sensitive nitrosamine analysis and support from a laboratory specialising in nitrosamines
As an analysis and expertise laboratory, FILAB offers:
- Ultra-trace nitrosamine analysis using our state-of-the-art equipment
- Characterisation of materials and inks to identify potential sources of contamination
- Comprehensive scientific support to guide you through regulatory procedures and audits
Our expertise enables you to respond quickly and effectively to FDA requirements.

