How FILAB helped one of its clients determine the different oxidation states of chromium using XPS in a powder?
Chromium is a chemical element belonging to the transition metals group. It is a hard, shiny, corrosion-resistant metal used in many fields due to its physical and chemical properties. Chromium has different oxidation levels.
During the manufacture of industrial parts for the aerospace industry, our client suspected the presence of chromium VI on a part coated with yellow zinc. After initial internal investigations, FILAB was asked to determine the presence of chromium VI on this coating and to quantify it.
Determination of the presence of Chromium VI by XPS
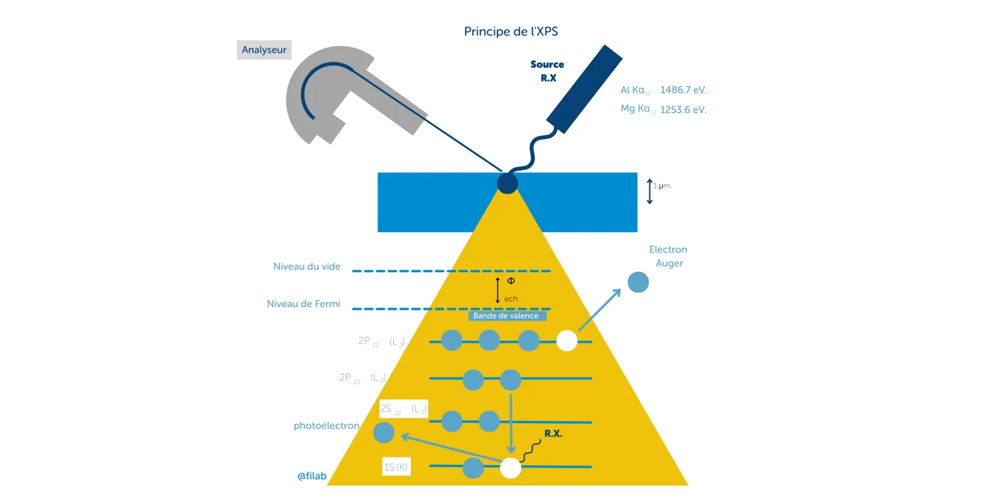
In order to carry out this study, the technique used is X-ray Photoelectron Spectroscopy (XPS), which allows for elemental, structural, and quantitative analysis of the extreme surface (a few nm deep) of a sample.
XPS is a quantitative chemical analysis technique with a depth of investigation in the nanometer range (typically between 2 and 4 nm). It can be applied to all solid samples that can withstand ultra-high vacuum (working pressure in the order of 10-8 Pa). It is therefore used in the case of the metal part that our customer wishes to have analyzed.
The sample is subjected to X-rays (generally Kα line of aluminum or magnesium). The X-rays excite the elements present in the sample. The result of the excitation can be the direct emission of an electron from a particular level (photoionization). XPS analysis consists of filtering by energy (using the analyzer) and detecting these photoelectrons.
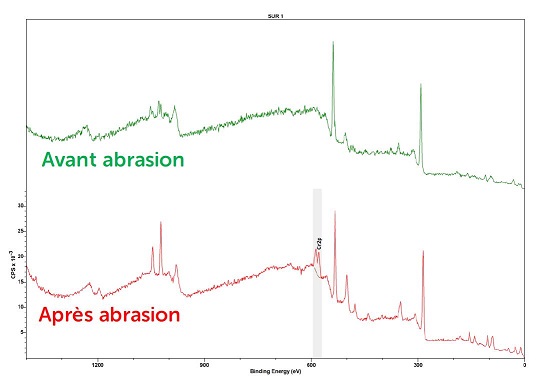
Abrasion was necessary to determine the presence or absence of chromium VI.
The initial analysis did not identify the presence of chromium in the sample due to the large amount of carbon on the sample's surface. We therefore abraded the sample's surface by 2-3 nanometers to remove this contamination.
XPS spectra of the sample – before abrasion (in green) and after abrasion (in red)
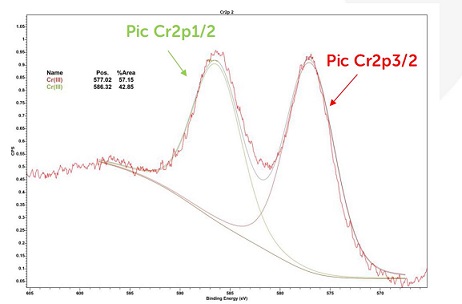
Deconvolution of the Cr2p peak allows the degree of chromium oxidation to be identified.
The energies of these contributions correspond to an oxidation state of +III. Indeed, the energies of the contributions linked to the oxidation state of +VI are located around 580 eV for the 2p3/2 peak and 589 eV for the 2p1/2 peak. These contributions are not present in the high-resolution spectrum of Chromium Cr2p.
High-resolution XPS spectra of Cr2p chromium from sample 2302-E0130013 after abrasion
The outer surface of the coated part therefore only contains trivalent chromium Cr (III). No traces of hexavalent chromium Cr (VI) were identified.
Why FILAB?
With significant experience in implementing Chrome VI analysis techniques, the FILAB laboratory can assist you with your needs:
- Chemical composition analysis and Chrome 6 (Chrome VI) testing
- Chromium 6 (chromium VI) testing on a glass sample
- Chromium 6 (chromium VI) testing on a part that has undergone nitrochromic treatment
- Determining the residual chromium 6 (chromium VI) content of surface treatments
- Chromium 6 (chromium VI) analysis on powder using UV spectrometry