Verification of the exhaustiveness of an extraction method in accordance with the ISO 19227 and ISO 10993-12 standards.
Your needs : to ensure the exhaustivity of the extraction method being used to validate your cleaning procedures in accordance with the ISO 19227 standard
Why verify the exhaustiveness of an extraction method ?
The ISO 19227 standard (Implants for surgery — Cleanliness of orthopedic implants — General requirements) specifies requirements for the cleanliness of orthopedic implants, and test methods for the cleaning process validation and controls, which are based on a risk management process. Particularly for THC and TOC analysis, as well as for analyzing detergents (using ICP) and acidic substances (using IC), the exhaustiveness of extraction methods must be proven in regards to the requirements of ISO 10993-12 (as is the case for chemical characterization in accordance with the ISO 10993-18 standard).
Our solution : to provide you with our skills relating to analysis and sample preparation method validation in accordance with the ISO 10993-12 standard
Our laboratory’s analytical services :
For over 30 years, FILAB laboratory has had the experience and specific analytical fleet to be able to assist companies from medical fields by providing analytical services and by verifying the exhaustiveness of extraction methods.
From analysis to expertise, FILAB laboratory is able to provide a wide range of analysis and method validation services for your Medical Devices :
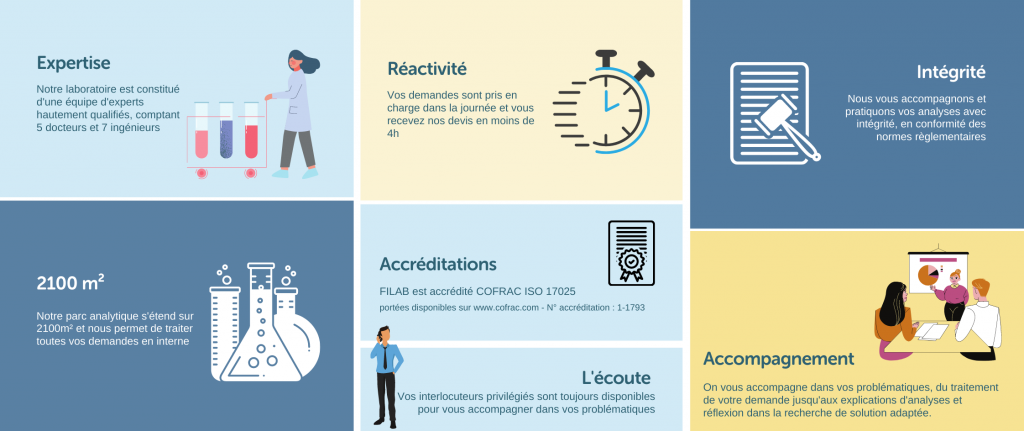
Why FILAB ?
As an independent laboratory with a team made up of highly qualified personnel, FILAB guarantees the reliability of its results, a quick turnaround for requests and tailored support for clients.
How to contact us ?
If you wish to call on our experts to perform analysis, you can request a free quote online here.
For more information about our analytical services, feel free to contact us via email at contact@filab.fr or over the phone by calling +33 (0)3 80 52 32 05
FILAB can also provide the following services :
- Biocompatibility testing in accordance with ISO 10993 (10993-18 / 10993-12 / 10993-13 / 10993-14 / 10993-15 / 10993-19 / 10993-22)
- R&D support : custom chemical analysis, material characterization, surface characterization, analytical development
- Problem solving : non-compliance, rupture, adhesive problem, corrosion…








