Differential Thermal Analysis (DTA) in the laboratory
You want to carry out a differential thermal analysis (DTA)
DTA analysis is used to measure the temperature variation between a sample and a reference during exo- or endothermic reactions. FILAB offers this technique to industry to characterise materials, optimise processes and ensure precise quality control.
DTA laboratory analysis
Differential thermal analysis (DTA) is a material characterization technique.
Differential thermal analysis (DTA) is a thermal analysis technique used to measure the temperature difference between a material sample and a reference material under the influence of specific conditions. These are temperature-controlled programmes in which both materials are subjected to the same heating or cooling conditions in a controlled environment.
The principles of DTA thermal analysis
Why perform an DTA analysis on a material?
Differential thermal analysis (DTA) is a method used in many industries to analyse materials and their properties, to help develop new products and to ensure quality control. Here are some examples of DTA applications in different industries:
- In pharmaceutical formulation, DTA is used to analyse the thermal stability of active ingredients and excipients. It can detect phase transitions such as melting temperature, recrystallisation or interactions between components, which can influence the efficacy and shelf life of drugs.
- DTA is used to characterize materials used in construction, such as cements and ceramics. It helps to identify thermal transformations such as hydration or thermal decomposition of compounds, which influence the quality and durability of materials.
- DTA analysis is also useful for determining the glass transition temperature of polymers, as well as for monitoring polymerisation and thermal degradation reactions. This information can be used to optimise manufacturing processes and improve the properties of plastic products.
In short, DTA can be used to characterize pharmaceuticals, foods, chemicals and inorganic substances.
You want to carry out a differential thermal analysis (DTA)
Differential thermal analysis (DTA) is a technique for the characterization of materials.
Differential thermal analysis (DTA) is a thermal analysis technique used to measure the temperature difference between a material sample and a reference material under the influence of specific conditions. These are temperature-controlled programmes in which both materials are subjected to the same heating or cooling conditions in a controlled environment.
The FILAB laboratory can help you with your differential thermal analysis (DTA) needs
As part of your industrial projects, FILAB offers you its expertise in Differential Thermal Analysis (DTA) and its cutting-edge equipment to meet your needs in the characterization of materials and the study of thermal phenomena.
Our technical resources for DTA analysis
FILAB offers high-performance DTA analysers that combine precision, robustness and versatility. These instruments allow rigorous temperature control over a wide range and flexible thermal programming, adapted to a wide variety of materials.
DTA analyser
DTA analyser
The DTA (Differential Thermal Analysis) analyser is used to study thermal transitions (melting, crystallisation, decomposition, etc.) by measuring the temperature difference between a sample and an inert reference when they are subjected to a controlled heating or cooling programme.
When the sample undergoes a thermal transition (melting, crystallisation, chemical decomposition), it absorbs or releases heat (endothermic or exothermic). These variations result in a temperature difference relative to the reference, which is recorded as a peak on a DTA curve.
Results obtained by differential thermal analysis
The DTA curve obtained represents the temperature difference as a function of temperature or time. Variations in the curve correspond to thermal transitions:
- Endothermic peak: Reactions requiring heat input (fusion, sublimation, thermal decomposition).
- Exothermic peak: Reactions that release heat (crystallisation, oxidation, polymerisation).
Other information is available after such an analysis :
- Characteristic temperatures :
- Start, end and peak temperature of thermal transitions.
- Identifying thermal transitions :
- Phenomena such as melting, crystallisation, vitrification or chemical reactions.
- Comparative analysis :
- ifferences between several batches, formulations or materials subjected to different conditions.
- Quality of materials :
- Detection of impurities or thermal defects (e.g. eutectic mixing or multiple transitions).
- Assessment of thermal stability :
- Thermal decomposition and identification of critical temperatures.
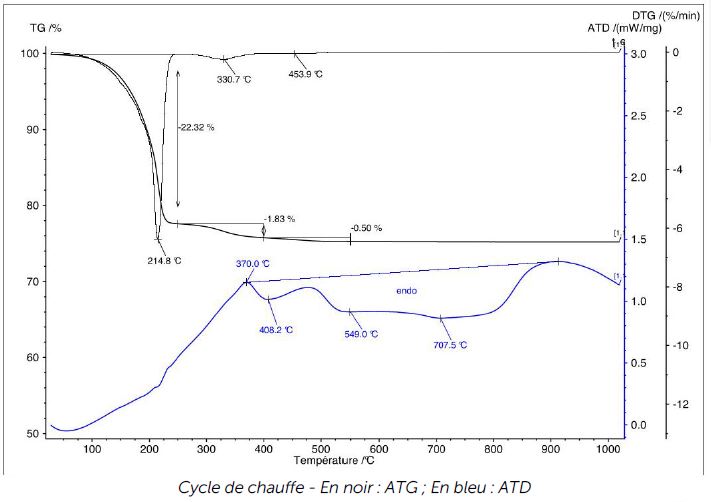
DTA and temperature measurements
DTA and qualification of thermal effects
Thermal effects play a key role in many industrial processes, from manufacturing to quality control. DTA is an essential tool for :
- Identify thermal interactions within materials (chemical reactions, phase transformations, etc.).
- Qualify the exothermic or endothermic phenomena involved in your production processes.
- Validate thermal behaviour for specific applications (behaviour in extreme environments, mechanical resistance under heat, etc.).
- Optimise product formulations based on their thermal response.
At FILAB, we offer a customised approach, integrating your specific needs for :
- Comparative studies between different batches or formulations.
- Monitoring the conformity of materials according to the industry standards.
- R&D for new product development: identification and control of critical thermal properties.
Other laboratory thermal analysis
How do I choose between DTA and DSC?
DTA detects thermal transitions (melting, crystallisation, etc.) by measuring the temperature difference between a sample and a reference. DSC goes further by precisely measuring the associated heat flows. DSC is recommended for quantitative analysis of thermal transformations.
DTA or ATG: which technique for which need?
DTA identifies thermal reactions with no change in mass, such as phase transitions. TGA measures mass losses due to decomposition. To study thermal stability or residues, TGA is essential.
DTA or DSC: what are the differences?
DTA identifies thermal events, while DSC accurately quantifies them. Choose DSC for detailed thermal analysis and DTA for a fast, robust initial diagnosis.
FAQ
Differential thermal analysis (DTA) is a technique used to analyse the thermal behaviour of materials. It measures the difference in thermal energy absorbed or released by a sample and an inert reference material during heating, cooling or temperature cycling. With DTA, our experts can measure phase transition temperatures, detect chemical reactions and compositional changes, and monitor kinetic parameters such as reaction rate constants. DTA analysis also helps to understand physical properties such as glass transitions, crystallisation processes and decomposition temperatures. The technique can be used for quality control and failure analysis by providing information on defects or impurities in materials. By analysing the response of a material to different thermal conditions, researchers gain information about its structure-function relationships and physical properties.
DTA analysis measures the heat flow between a sample and a reference material. The two materials are placed in an instrument where they are heated or cooled together at a uniform rate. A thermocouple is used to measure the temperature difference between the two materials, which is recorded over time. By tracing this record of thermal activity, our teams can identify events such as phase transitions, glass transitions, crystallisation processes and chemical reactions that occur during the heating or cooling of the sample.
Differential thermal analysis (DTA) is a powerful technique for analysing the behaviour of materials at different temperatures. It provides essential information on compositional changes and physical properties of samples. This information can be used for quality control and failure analysis. Filab can assist you with your thermal analysis needs.
DTA analysis should be performed if you need to understand the behaviour of your sample under different thermal conditions or if you want to identify impurities or defects. It is often used in failure analysis to determine the root cause of product failures. For example, differential thermal analysis can be used to analyse how the structure and composition of a material changes when exposed to high temperatures. Filab's experts are ready to help you with your DTA needs!
During differential thermal analysis (DTA), several properties of materials are measured.
DTA detects and quantifies temperature changes between a sample and a reference material, revealing events such as phase transitions (melting, crystallisation), chemical transformations (decomposition, oxidation) and heat absorption or release reactions.
These data can be used to identify the specific temperatures at which these events occur, providing valuable information about the thermal stability, chemical composition and physical properties of the materials studied.
This method can be used to study phenomena such as phase transitions, glass transition, polymerisation, crystallisation, melting and sublimation. By heating or cooling the sample and reference material at a uniform rate and measuring the temperature difference with a thermocouple, it is possible to record thermal activity. This allows events such as phase transitions, crystallisation processes and chemical reactions to be identified.
Differential thermal analysis (DTA) is used to observe different types of reactions and thermal transitions in materials.
- Phase transitions are changes in the physical state of a material from solid to liquid, playing a crucial role in assessing the purity and thermodynamic properties of materials.
- Glass transition is a change in molecular mobility, indicating when a material becomes less rigid and more flexible (polymer).
- Thermal decomposition and oxidation reactions involve chemical degradation and interaction with oxygen, affecting the stability and durability of materials respectively.
- Adsorption and desorption reactions, which involve the exchange of gases or vapours, influence the surface properties and chemical reactivity of materials.
- Sublimation and vaporisation describe the transition from a solid or liquid state to a gaseous state, providing insight into the volatility and thermal stability of compounds.
- Crystallisation and recrystallisation refer to the formation or modification of crystalline structures, especially for materials dependent on their crystalline properties.
- Exothermic and endothermic reactions, which release or absorb heat, are important for thermal energy management and the safety of industrial processes.
The choice between DTA and DSC depends on the objectives of the analysis and the properties of the material to be studied.
DTA is ideal for qualitative studies, making it possible to detect thermal transition temperatures and identify phenomena such as endothermic or exothermic reactions, without quantifying the heat exchanged. It is suitable for materials with clear, well-defined transitions.
DSC analysis, on the other hand, is recommended for precise quantitative analysIs, measuring heat flows to determine the transition enthalpies and energies associated with thermal phenomena. More sensitive and precise, DSC is essential for studying the purity, thermal stability or energetic properties of complex materials such as polymers or mixtures.
The choice therefore depends on the level of precision required and the type of data needed for the application.








